MimetikOss® 3D
Custom-made biomimetic synthetic bone graft

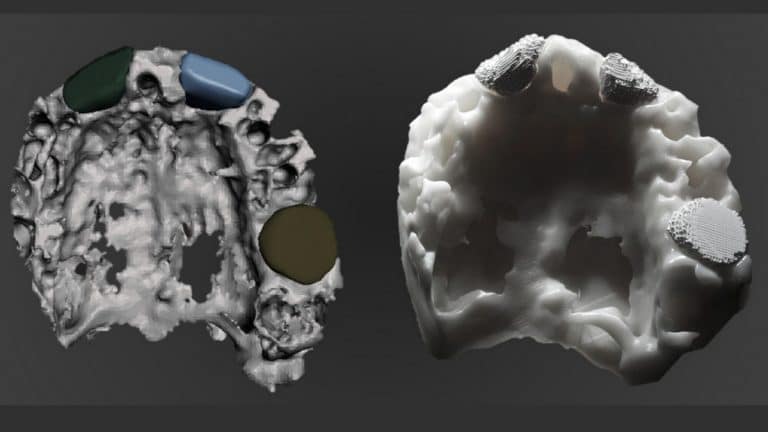

Mimetis engineers design a 3D printed bone based on the medical images of the patient, to perfectly fit the anatomy.
The biomimetic synthetic bone graft is not sintered. In other words, we do not subject it to any extreme temperatures hardening process, its structure and composition are similar to the bone mineral phase, exhibiting a high specific surface area and multi-scale porosity.This is why MimetikOss® 3D is perfect for bone augmentations of complex geometries.
Features
Composition
MimetikOss® 3D is composed of β-tricalcium phosphate (≤ 40%) and of biomimetic hydroxyapatite (≥ 60%).
100% customized
Overall, MimetikOss® 3D is a biomimetic synthetic bone graft that is custom made. In other words, it is based on the anatomy of the patient. Thus, this allows a perfect fit of the graft with the bone defect even for complex geometries.
Biomimetic
Biomimetic calcium deficient hydroxyapatite and β-TCP. This is why the body recognizes it as the patient’s own bone, so the use of the graft presents a low risk of rejection or infection.
Highly porous
The nano-, micro- and macro-porosity favors the adsorption of proteins and cell adhesion. Therefore, it facilitates the colonization of the graft by bone cells. Also, it supports the vascularization and the growth of newly formed bone.
At the macrometric level, the adjustments of the impression allow to obtain an open and interconnected porosity that favors the vascularization and the colonization of the tissues.
Highly controlled
During the manufacturing process, the precise control of the architecture of the porosity. Specifically, the size of the interconnected network of pores. Further, it allows adapting the response of the body to the bone graft.
Structural integrity
MimetikOss® 3D adapts exactly to the anatomy of the patient. However, and if strictly necessary, the surgeon can further modify the graft during surgery, with a surgical handpiece or other cutting tools.
Indications
MimetikOss® 3D is designed for the regeneration of the following osseous defects:
- Cranio-maxillofacial/dental
- Orthopedic.
MimetikOss® 3D is indicated for non-load bearing applications but its fixation with osteosynthesis material can open its applications to load-bearing indications.
MimetikOss® Granules
Biomimetic synthetic option
We at Mimetis manufacture MimetikOss® under hydrothermal processes that mimic the natural bone’s structure and composition.
Indeed, MimetikOss® Granules is the 4th and latest generation biomimetic synthetic bone graft that allows creation of a highly vascularized bone tissue. Furthermore, it integrates exceptionally well with the surrounding soft tissue, due unquestionably to its nano-, micro- and macro-porosity.
As a result, not only does it preserve bone volume, but it also activates natural bone regeneration. Therefore, MimetikOss® covers the most common indications up to the most challenging ones.
Features
Composition
To point out, MimetikOss® is a biomimetic synthetic bone graft on the market. Indeed, it is similar in shape, structure and composition to the mineral phase of human bone. The reasons for that include:
- 80% biomimetic calcium-deficient hydroxyapatite (CDHA)
- 20% biomimetic beta-tricalcium phosphate (β-TCP)
Sizes
MimetikOss® is readily available in two sizes:
- S (0.2 – 1 mm)
- M (1 – 2 mm)
Biomimetic
MimetikOss® replicates the structure and composition of the mineral part of the bone. Consequently, it’s recognized as natural bones by the bone cells
Radiopacity
In particular, MimetikOss®’ radiopacity is similar to natural bone’s. The reason for that is its similarity in structure and composition.
Easy to use
Most importantly, MimetikOss® is particularly highly hydrophilic for easier user-handling.
Volume stable
In the same way, MimetikOss®’ spheroidal morphology avoids stacking over time.
Indications
- Augmentation or reconstruction treatment of the alveolar ridge.
- Filling of infrabony periodontal defects.
- Filling of defects after root resection, apicoectomy and cystectomy.
- Filling of extraction sockets to enhance preservation of the alveolar ridge.
- Elevation of periodontal defects in conjunction with products intended for Guided
- Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR).
- Filling of peri-implant defects in conjunction with products intended for Guided
- Bone Regeneration (GBR).
